Our Research Focus Areas
Understanding the role of fibroblast specific protein-1 (S100A4) in pro- fibrotic and inflammatory pathways in endometriosis
This study looks into the aspect of endometriosis which is characterized by occurrence of fibrosis and myofibroblasts within endometriotic lesion. Moreover, S100A4 has been implicated in the pathogenesis of fibrotic, inflammatory, and autoimmune conditions. They may play a role in promoting the migration and invasion of endometrial cells in peritoneal cavity. It further can act as a crucial mediator of the interplay between fibrosis and inflammation by altering the extracellular matrix components and remodelling enzymes, driving the increase of fibrotic markers as well as profibrotic genes. However, recent findings have suggested that M2 macrophages are dominant in endometriotic lesions. Therefore, the proposal takes a closer look at the role of macrophages as a contributor of extracellular S100A4 in endometriosis development and fibrosis progression. Furthermore, we want to test if pharmacological inhibition can improve endometriosis associated fibrosis.
Artificial Intelligence augmented oocyte retrieval for improved outcome of Assisted Reproductive Technology (AI4ART)
The presence of endometriomas poses significant challenges during the in-vitro fertilization (IVF) process, making it difficult to collect oocytes from the ovary. Currently, there is no effective way to prevent accidental contamination of aspirated oocytes with endometriotic tissues while performing Transvaginal Oocyte Retrieval (TVOR) in endometriotic patients. To address this issue, we propose the development of a multispectral optoacoustic and ultrasound imaging-based smart needle guiding technology for oocyte retrieval. By integrating artificial intelligence (AI) and cutting-edge imaging techniques, our aim is to automate the oocyte retrieval process and minimize risk factors during assisted reproduction. The guided intervention involves identifying the boundaries of endometriomas and follicles with viable oocytes, which is enhanced by utilizing multispectral imaging with AI-assisted identification based on relevant clinical biomarkers. The real-time imaging information will then guide the interventionalist to avoid potential contamination of extracted oocytes. The proposed system will calculate the optimal trajectory to avoid touching the endometrioma and non-mature oocyte walls, providing visual assistance to the operator. The findings will be validated using standard laboratory protocols for IVF on the extracted oocytes.
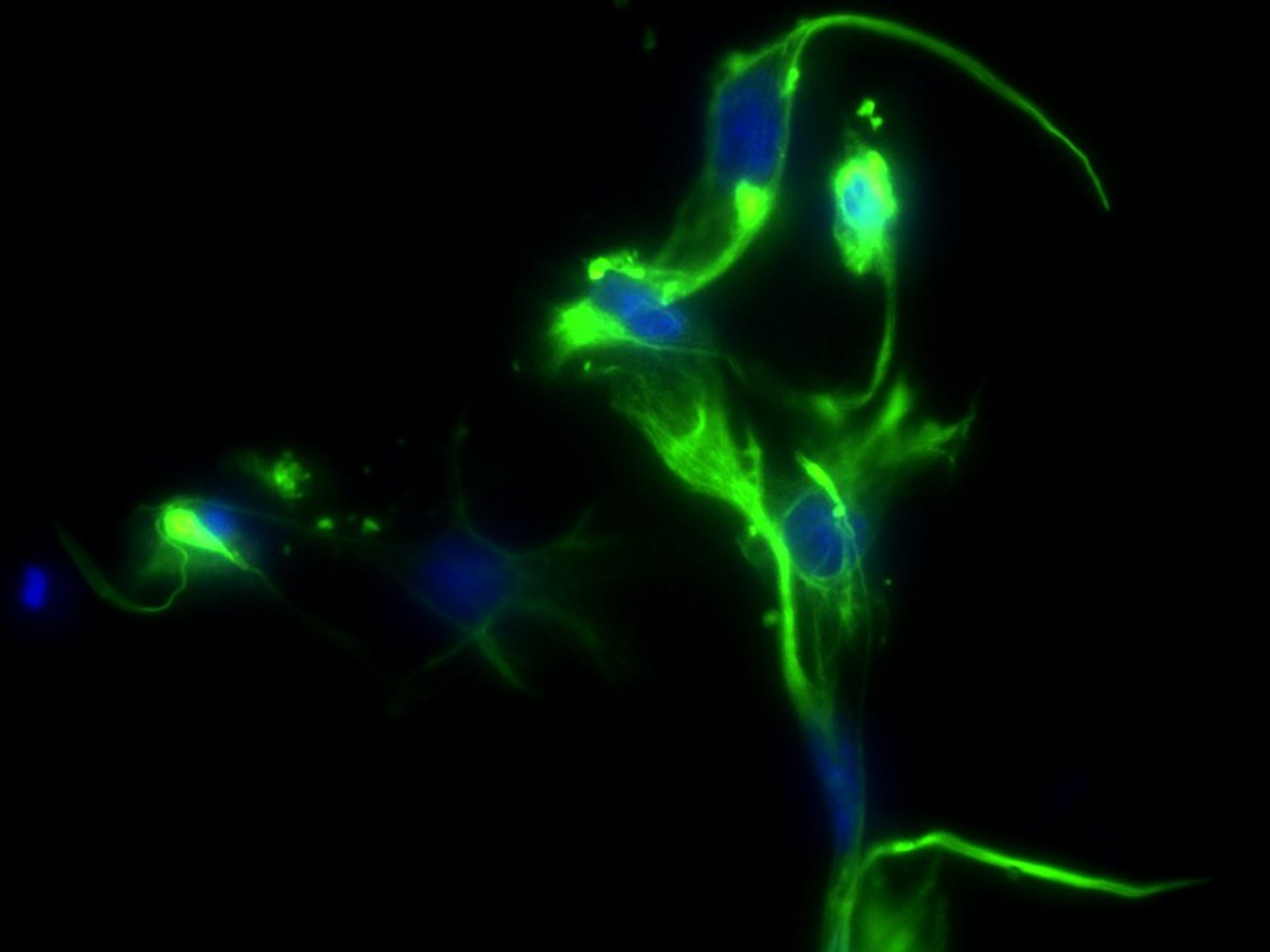
Understanding the molecular dynamics of activated macrophages and neutrophils in endometriosis microenvironment
The study addresses the impact of macrophage and neutrophil functionality within the endometriotic milieu, elucidating immunological dysregulation that may facilitate disease development. We intend to develop a syngeneic mouse model that more accurately mimics the recurrent tissue injury and repair (ReTIAR) process, offering a more physiologically relevant framework for investigating fibrosis and immunological interactions. A fundamental component of this research involves the targeted depletion of M2 macrophages through a peptide-based methodology to evaluate its therapeutic efficacy. The research combines in vivo and in vitro models to enhance the understanding of immune regulation in endometriotic lesions. Findings from this research strengthen disease modeling and investigate targeted therapies that may result in strategies for novel therapeutic targets for managing endometriosis.
Investigate: To conduct rigorous, ethical, and innovative research into the biological, psychological, and socio-cultural factors underlying menstrual pain and endometriosis in the Indian population.
Understand: To elucidate the diverse mechanisms and experiences of dysmenorrhea and the complex pathophysiology of endometriosis.
Innovate: To explore and validate novel diagnostic tools, particularly non-invasive methods for early endometriosis detection, and effective therapeutic strategies for managing menstrual pain and endometriosis.
Collaborate: To foster partnerships with researchers, clinicians, policymakers, patient advocacy groups, community organizations, and industry to accelerate progress in menstrual health and endometriosis care.
Educate & Advocate: To raise awareness, combat stigma associated with menstrual issues and endometriosis, and disseminate evidence-based information to the public, healthcare providers, and affected individuals.
Our Vision
To be a leading national and international centre for research on menstrual pain and endometriosis, driving scientific discovery and translating knowledge into timely diagnosis, effective management strategies, and accessible, culturally sensitive solutions that alleviate suffering and empower individuals.
Our Mission
Why this Research Matters?
Prevalence & Impact: Menstrual pain is common, but conditions like endometriosis cause chronic, debilitating pain extending beyond menstruation, profoundly impacting quality of life.
Diagnostic Delays: Endometriosis often faces significant diagnostic delays (average 7-10 years globally), leading to prolonged suffering, disease progression, and potential complications like infertility. Our research aims to shorten this diagnostic journey.
Beyond 'Bad Periods': Investigating conditions like endometriosis is crucial to differentiate severe underlying pathology from common menstrual cramps, ensuring individuals receive appropriate and timely care.
Research Gap in India: There is a profound lack of India-specific data on the prevalence, presentation, underlying causes, risk factors, and optimal management for both general menstrual pain and specifically for endometriosis.
Health Equity: Addressing menstrual pain and endometriosis effectively is essential for advancing women's health, well-being, and gender equity.